

Diffusion constants are given for several gases in waterĭry Air and Water Vapor - Density and Specific Volume vs. Diffusion flux tells how fast a substanse solved in another substance flows due to concentration gradients. Specific volume, enthalpy and entropy of compressed water Hot and cold water service systems - design properties, capacities, sizing and more
Steam & condensate systems- properties, capacities, pipe sizing, systems configuration and more Effects of work, heat and energy on systems Material properties for gases, fluids and solids - densities, specific heats, viscosities and more

Specific enthalpy and entropy for liquid water at temperatures from 32 to 675 ☏:Įnergy per unit mass (Specific energy - mass)īritish thermal unit(international table)/pound, gigajoule/tonne, kilocalorie/kilogram = calorie/gram, kilojoule/kilogram = joule/gram, kilowatt hour/kilogram
STANDARD ENTROPY TABLE FULL
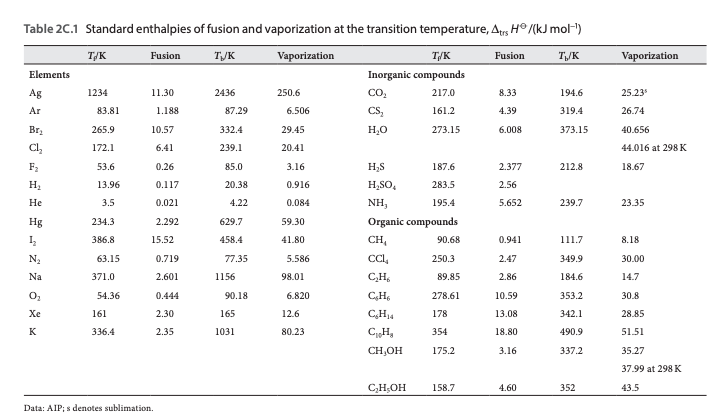
Specific enthalpy and entropy for liquid water at saturation pressure at temperatures from 0 to 374 ☌:įor full table with Entropy - rotate the screen! Temperature See also Water Boiling points at high pressure, Boiling points at vacuum pressure, Density, specific weight and thermal expansion coefficient, Dynamic and kinematic viscosity, Heat of vaporization, Ionization Constant, pK w, of normal and heavy water, Melting points at high pressure, Saturation pressure, Specific gravity, Specific heat (heat capacity) and Specific volume for online calculatores, and similar figures and tables as shown below. See Water and Heavy Water - thermodynamic properties. The figures and tables below shows how water enthalpy and entropy changes with temperature (☌ and ☏) at water saturation pressure (which for practicle use, gives the same result as atmospheric pressure at temperatures < 100 ☌ (212☏)). Compounds of the transuranium elements are not included.Follow the links for definitions of the terms specific enthalpy and entropy. Values are not given for alloys or other solid solutions, fused salts or for substances of undefined composition. Values are given for properties of gaseous, liquid and crystalline substances, for solutions in water, and for mixed aqueous and organic solutions.
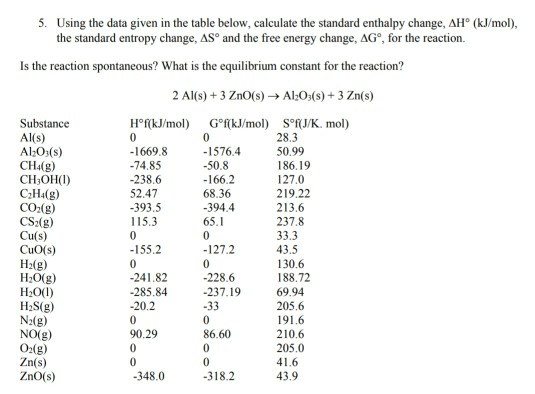
This volume is a new collective edition of "Selected Values of Chemical Thermodynamic Properties," which was issued serially as National Bureau of Standards Technical Notes 270-1 (1965) to 270-8 (1981). All values are given in SI units and are for a standard state pressure of 100 000 pascal. Where available, values are given for the enthalpy of formation, Gibbs energy of formation, entropy, and heat capacity at 298.15 K (25 degrees C), the enthalpy difference between 298.15 and 0 K and the enthalpy of formation at 0 K. Recommended values are provided for chemical thermodynamic properties of inorganic substances and for organic substances usually containing only one or two carbon atoms.


 0 kommentar(er)
0 kommentar(er)
